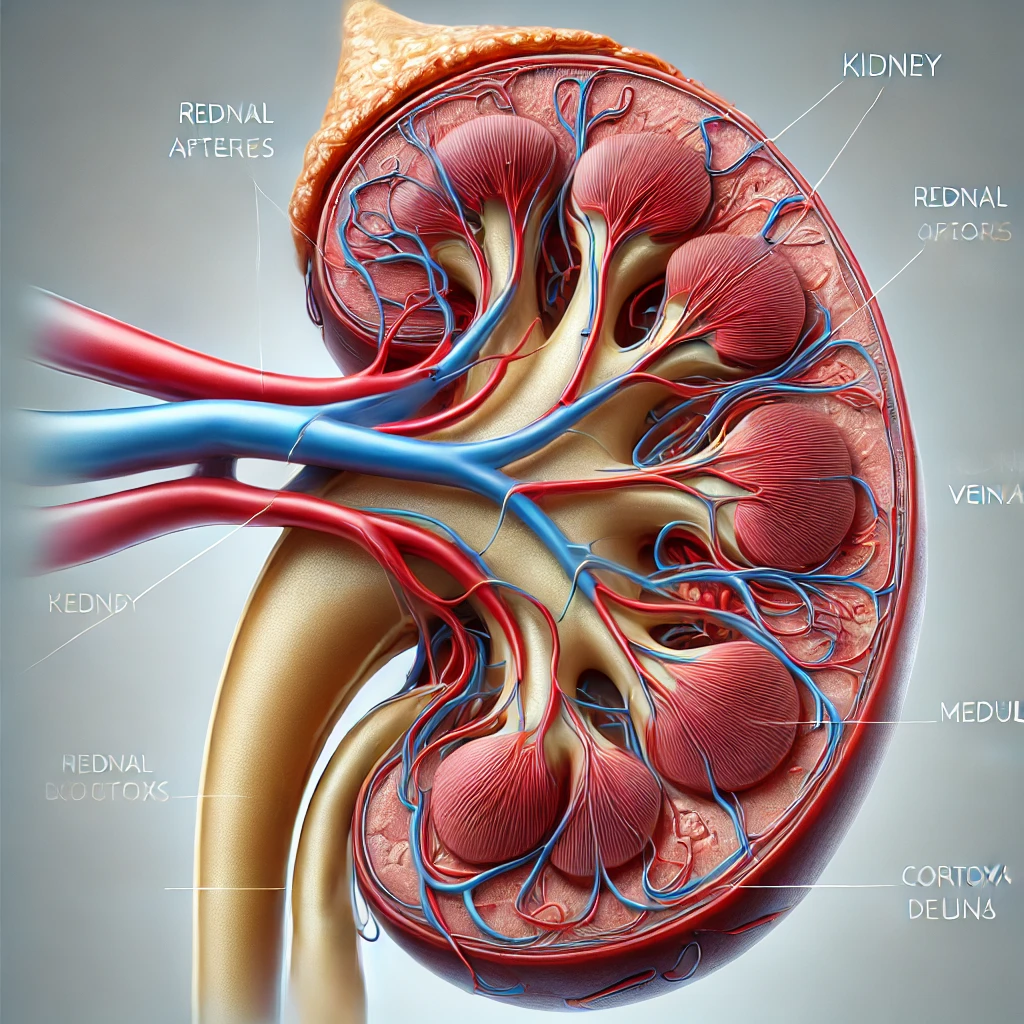
Chronic Kidney Disease (CKD) Diagnosis in Elderly Patients: Understanding eGFR Interpretation and Age-Specific Cutoffs
1. Introduction: The Growing Burden of CKD in an Aging Society Chronic Kidney Disease eGFR interpretation in elderly patients, With the rapid aging of populations worldwide, the prevalence of chronic kidney disease (CKD) among elderly… Chronic Kidney Disease (CKD) Diagnosis in Elderly Patients: Understanding eGFR Interpretation and Age-Specific Cutoffs