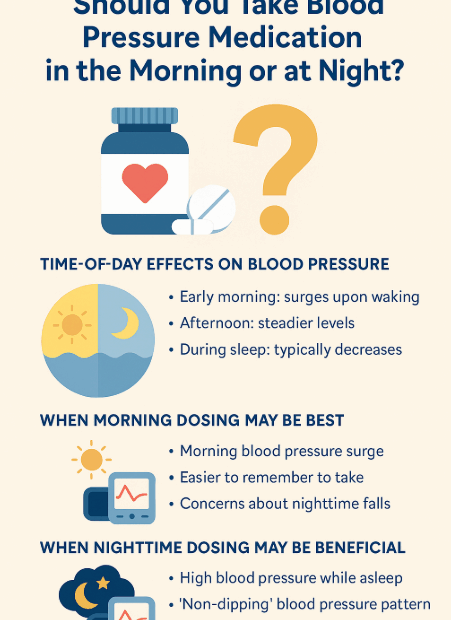
Should You Take Blood Pressure Medication in the Morning or at Night?
✔ Introduction: Timing Matters in Hypertension Control If you take medication for high blood pressure, you’ve probably wondered: “Should I take my medication in the morning? Or would it work better at night?” This is… Should You Take Blood Pressure Medication in the Morning or at Night?